Individualized Medication Guidance for Gynecologic Cancer
Quick Detail
- Minimum order:1
Specifications
Gynecologic Cancer Surgery
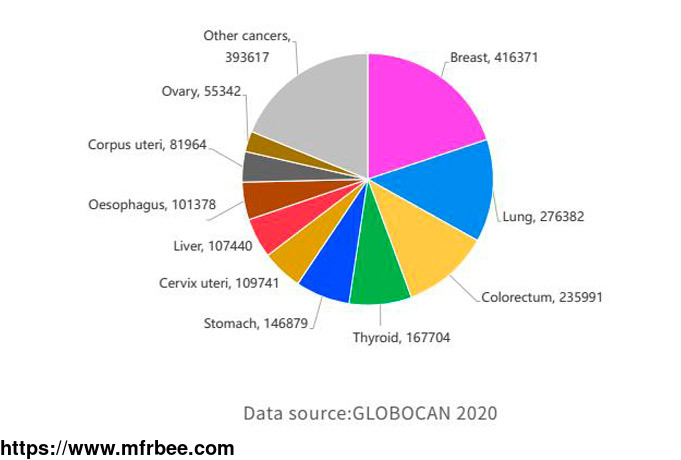
According to the latest Chinese women\'s new cancer statistics, cervical cancer and ovarian cancer are in the top ten new cases of female cancer (about 110,000 and 60,000 respectively). With the
widespread clinical use of poly(ADP-ribose) polymerase (PARP) inhibitors, the progression-free survival (PFS) of ovarian cancer patients has been significantly prolonged. At present, the biomarkers
related to PARP inhibitor treatment mainly include BRCA1/2 gene mutation, HRR gene mutation, etc., and some studies have found that about 50% of epithelial ovarian cancer patients have homologous
recombination deficiency (homologous recombination deficiency, HRD).
Test Content of Individualized Medication Guidance for Gynecologic Cancer Care
This test detects 348 genes, including HRR pathway genes. The content involves targeted therapy, immunotherapy, chemotherapy and hereditary risk assessment, and provides comprehensive medication
reference for gynecologic cancer patients.
Applicable Population of Individualized Medication Guidance for Gynecologic Cancer Treatment
Late-stage newly diagnosed gynecologic cancer patients who need targeted therapy and immunotherapy.
Patients with relapsed, drug-resistant gynecologic cancer solution.
Gynecologic cancer patients who need targeted therapy and immunotherapy through liquid biopsy.
If you want to know more details of chemotherapy in gynaecology, radiotherapy in gynaecology, please visit our website.
As one of genetic sequencing companies, we can provide professional chip seq service for clients, if you are interested, please leave us a message.