Bacterial Vaginosis Rapid Test Kit core Chromogenic Ingredient IBX-4041
Quick Detail
- Minimum order:1
- Place of Origin:China
Specifications
Core Chromogenic Ingredient IBX-4041 for Bacterial Vaginosis BV Blue Rapid Test Kit
Bacterial Vaginosis Rapid Test Kit core Chromogenic Ingredient IBX-4041
IBX-4041 Testing vessel
BV Blue active ingredient
BVBlue active ingredient
BVBlue substrates
BV Blue substrates
BVBlue® substrates
BVBlue™ substrates
Chromogenic substrate IBX-4041
chromogenic substrate of sialidase enzyme
Detection reagent for bacterial vaginosis (sialidase two-step method)
Shenzhen BST Science & Technology Co., Ltd sells and exports chromogenic substrate of sialidase enzyme IBX-4041.
Specification:
Appearance: Light yellow solid powder
Purity: 95% min
Application: Two-step chromogenic substrates of neuraminidase (sialidase)
Storage: 2-8℃, keep Away from Moisture and light
Packaging details: 1g per bottle or according to customer’s requirement
BVBLUE Test Kit Component
1)BVBLUE Developer Solution: Water and Sodium hydroxide
2) BVBLUE Testing Vessel: Water, Potassium acetate, IBX-4041 (chromogenic substrate compound).
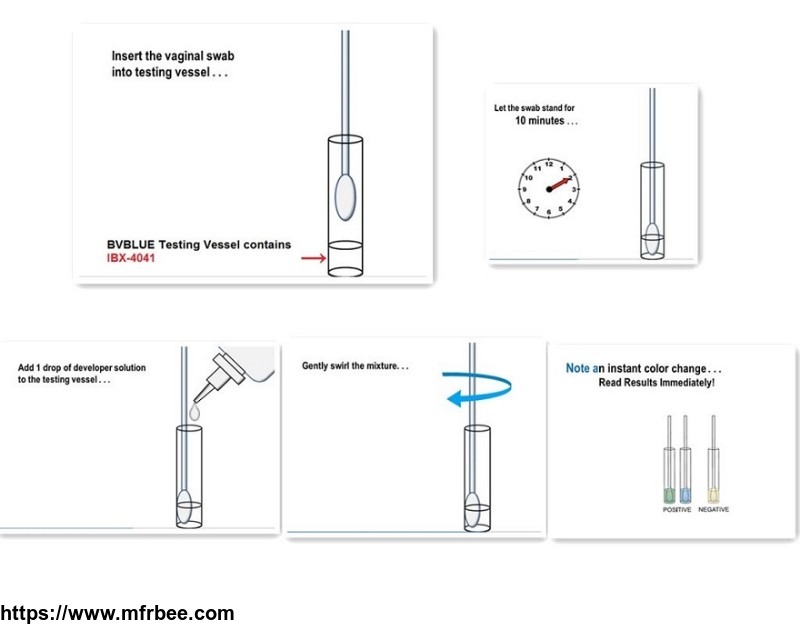
IBX-4041 is the key ingredient of BVBLUE test kit, BVBLUE test is a rapid sialidase test for bacterial vaginosis. BVBLUE test method is currently the only test method approved by the FDA for testing Bacterial Vaginosis(BV), The BV BLUE Test contains a chromogenic substrate of sialidase enzyme, IBX-4041.
BV Blue test is a commercially available test, it detects sialidase activity, an enzyme produced by BV-associated bacteria such as Gardnerella vaginalis, Bacteroides spp., Prevotella spp., and Mobiluncus spp. In the test procedure, a vaginal fluid sample is placed in the test vessel which contains a chromogenic substrate for sialidase. After incubation, a developer solution is added, and If the sample contained a high level of sialidase, a blue or green color is seen. Samples containing no sialidase, or low levels of this enzyme, will generate a yellow color in the reaction. The BV test has high specificity and sensitivity. The BVBlue™ test enables rapid detection of BV in a physician\'s office, and can thereby provide advantages over the Amsel methods and other methods requiring extensive laboratory testing.
Advantage of BVBLUE Test Kit
Compared to other diagnostic test methods for BV such as Amsel criteria, Nugent Gram-stain scoring system, Affirm® Microbial Identification Test, BVBLUE Test Kit has following advantage:
1, BVBLUE Test is currently the only test method approved by the FDA for testing Bacterial Vaginosis(BV).
2, BVBLUE Test just take 10 minutes to 15 minutes to get the result.
3, High purity sialidase substrate with high specificity can reduce the occurrence of false positive.
4. Comparing with the gold standard, the coincidence rate is high, the relative sensitivity is 93%, and the specificity is 95%.
5. Easy to operate, do not need equipment.
6. The specimen does not need to pretreat.